Our eosinophilosophy on
WHY EOS COUNTS
Eosinophils are multifunctional leukocytes that can be both helpful and harmful, making their role difficult to determine.1,2 While eosinophil involvement in inflammatory disease is well-established, more is being understood about their role in health and how they can help guide diagnosis and treatment decisions.1,3-10
The role of eosinophils in health was long thought to be limited to defense against parasitic infections. It is now known that eosinophils could contribute to overall health and immunoregulation.2,11
Contribution to overall health and immunoregulation comes from both blood- and tissue-resident eosinophils
In fact, many blood- and tissue-resident eosinophils deliver protective effects in response to infections and disease. In addition to protection against parasitic infections, eosinophils are thought to play a role in host defense of some viral, bacterial, and fungal infections.11,12 Not only do eosinophils directly participate in innate immunity, they may have many roles in adaptive immunity, including regulation of T-cell development in the thymus, lymphocyte recruitment, antigen presentation, and driving T helper 2 (Th2) cell polarization.13 Resident eosinophils in the gastrointestinal tract have been shown to contribute to intestinal immune homeostasis by regulating T-cell and immunoglobulin A responses.14,15
Tissue-resident eosinophils are involved in tissue maintenance, metabolism, and immune homeostasis; however, their specific function depends on the tissue in which they reside.12,14 Under physiologic conditions, tissue-resident eosinophils have been identified in14:
Adipose tissue
Gastrointestinal tract
Lung
Mammary gland
Spleen
Uterus
Eosinophils may also play a role in tissue repair through secretion of fibroblast growth factor and transforming growth factor-β. These growth factors are secreted from the eosinophil following epithelial damage.13

A recent study found mean blood eosinophil levels in a healthy population (n=3641) to be 107 eosinophils/μL. The same study found blood eosinophil counts are consistently higher in males (120 cells/μL median) than females (100 cells/μL median), are highest in infancy and adolescence, and are independent of age in adults. Additionally, atopy, smoking, asthma, chronic obstructive pulmonary disease, obesity, and metabolic syndrome can be associated with higher eosinophil counts.16*
*Geometric mean; 95% confidence interval (105-110 eosinophils/μL).
Although eosinophils may contribute to overall health, the impact of levels
below what is considered normal is not known11
During immune responses mediated by Th2 cells and group 2 innate lymphoid cells (ILC2), eosinophils become activated and multiply. Th2 cells and ILC2 cells are major sources of interleukin 5 (IL-5), the main cytokine responsible for maturation, activation, and survival of eosinophils.12,17 Eosinophils release cytokines and chemokines to target foreign antigens, promote inflammation, and damage surrounding structures.1,12,13
Cytokines and chemokines released from eosinophils lead to inflammation and tissue damage12,17,18
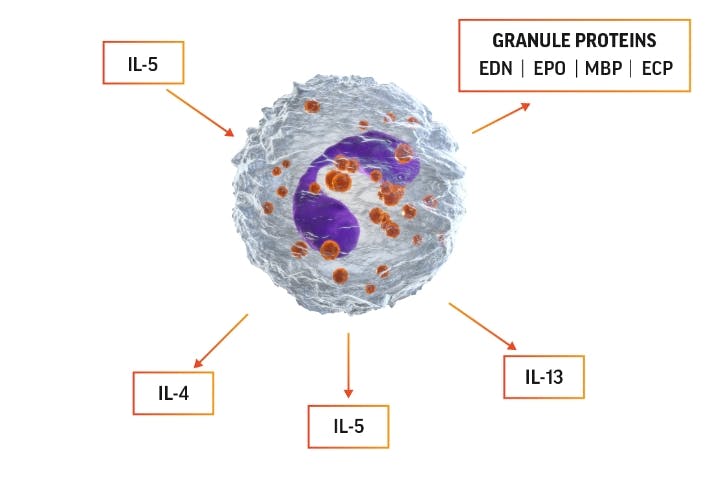
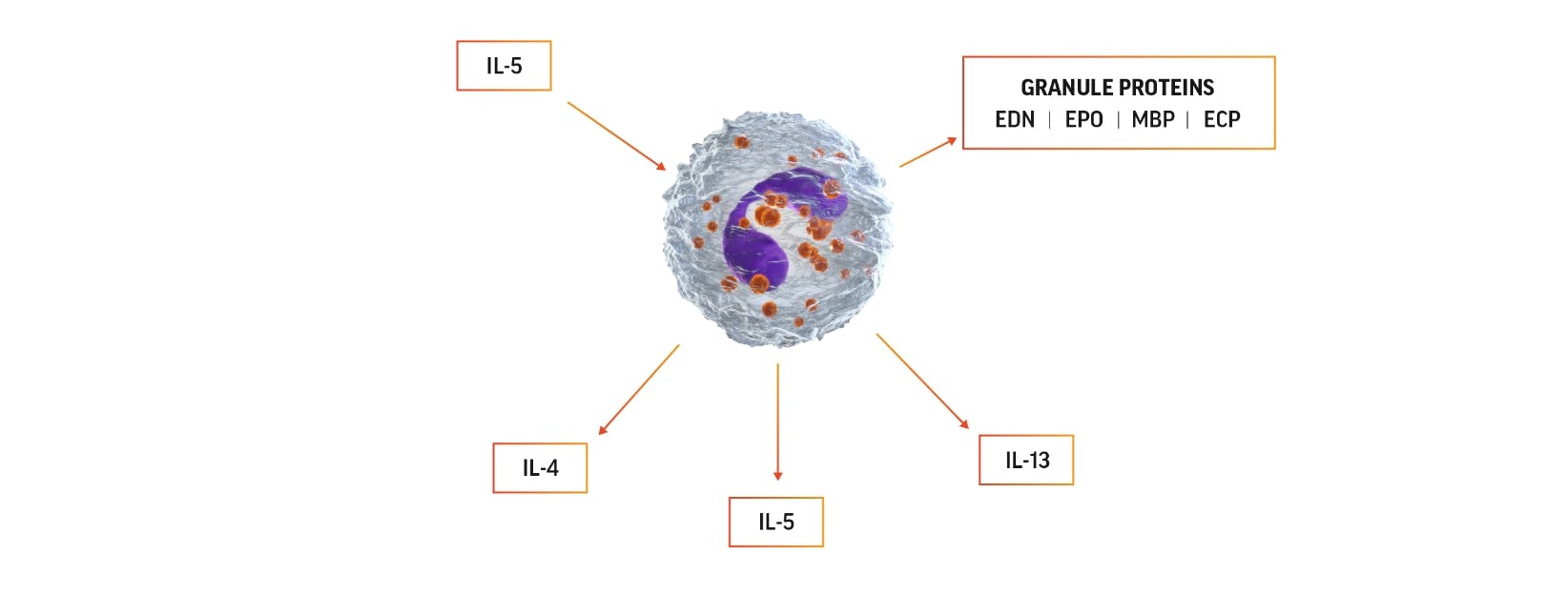
Eosinophil activation can lead to production of IL-5, which can promote
eosinophils’ own survival by eosinophilopoiesis and activation.
The 4 major proteins released from eosinophil granules and their functions include12:
Eosinophil cationic protein (ECP)
- Causes membrane disruption
- Cytotoxic to host cells and pathogens
- Neurotoxic
Eosinophil-derived neurotoxin (EDN)
- Antiviral
- Neurotoxic
- Maturation, activation, and chemotaxis of dendritic cells
Eosinophil peroxidase (EPO)
- Leads to generation of reactive oxygen species, which are toxic to extracellular pathogens
- Inflammatory effects
- Anti-inflammatory effects
Major basic protein (MBP)
- Causes membrane disruption
- Cytotoxic to host cells and pathogens
- Activates mast cells, basophils, and neutrophils
- Neuroprotective effects
Reconsider the role of IL-5 in eosinophilic disease. Watch the video below to learn more about the impact of IL-5, eosinophils, and other inflammatory cells in type 2 inflammation.
Blood and/or tissue eosinophils can be used as a biomarker in clinical practice to guide diagnosis and treatment. Your patients’ blood eosinophil levels can be measured through a complete blood count with differential.3-10
References:
- Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17(12):746-760.
- Travers J, Rothenberg ME. Eosinophils in mucosal immune responses. Mucosal Immunol. 2015;8(3):464-475.
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2024 main report. https://ginasthma.org/2024-report/. Accessed July 25, 2024.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2024 report. https://goldcopd.org/2024-gold-report/. Accessed July 25, 2024.
- Asano K, Hebisawa A, Ishiguro T, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2021;147(4):1261-1268.e5.
- Wechsler ME. Pulmonary eosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27(3):477-492.
- Suzuki Y, Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019;68(4):413-419.
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990;33(8):1094-1100.
- Reed CC, Dellon ES. Eosinophilic esophagitis. Med Clin North Am. 2019;103(1):29-42.
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130(3):607-612.e9.
- Klion AD, Ackerman SJ, Bochner BS. Contributions of eosinophils to human health and disease. Annu Rev Pathol. 2020;15:179-209.
- Ramirez GA, Yacoub MR, Ripa M, et al. Eosinophils from physiology to disease: a comprehensive review. Biomed Res Int. 2018;2018:9095275.
- Wen T, Rothenberg ME. The regulatory function of eosinophils. Microbiol Spectr. 2016;4(5):10.1128/microbiolspec.MCHD-0020-2015.
- Marichal T, Mesnil C, Bureau F. Homeostatic eosinophils: characteristics and functions. Front Med (Lausanne). 2017;4:101.
- Chu DK, Jimenez-Saiz R, Verschoor CP, et al. Indigenous enteric eosinophils control DCs to initiate a primary Th2 immune response in vivo. J Exp Med. 2014;211(8):1657-1672.
- Hartl S, Breyer MK, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. 2020;55(5):1901874.
- Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303-1309.
- Varricchi G, Senna G, Loffredo S, Bagnasco D, Ferrando M, Canonica GW. Reslizumab and eosinophilic asthma: one step closer to precision medicine? Front Immunol. 2017;8:242.
