ONSCREEN TEXT:
Exploring the Importance of Type 2 Inflammation and Eosinophils in COPD
DR. CRINER:
Greetings and welcome to “Exploring the Importance of Type 2 Inflammation and Eosinophils in COPD”.
My name is Gerard Criner, and I’m a pulmonologist and chair of Thoracic Medicine and Surgery at Temple University in Philadelphia.
As you know, this is a very exciting time in pulmonary medicine as our understanding of COPD continues to evolve.
We are going to discuss the critical unmet needs in COPD, explore the contributions of type 2 inflammation in driving disease progression, and review the role of blood eosinophils in patient identification.
ONSCREEN TEXT:
GSK’s Commitment to Transparency and Education
This program is being provided to you today by an external expert contracted with GSK. This external expert has been provided an honorarium for this event.
GSK is committed to transparency of financial relationships with healthcare professionals
DR. CRINER:
Before we begin, I want to mention that this program is being sponsored by GSK, and I am receiving compensation for this event.
This discussion will focus on disease education only. Product selection and patient treatment will not be addressed.
ONSCREEN TEXT:
What are the most urgent unmet needs that we face when managing patients with COPD and frequent exacerbations?
COPD=chronic obstructive pulmonary disease.
DR. CRINER:
As clinicians who manage patients with COPD, our top priority is often reducing exacerbations. For many of our patients, this can be quite difficult.
Let’s begin our discussion with a deeper look into the challenges related to frequent exacerbations in our patients with COPD.
ONSCREEN TEXT:
COPD Is a Progressive Inflammatory Lung Disease Impacting Approximately 14 Million Americans1
COPD is a heterogeneous disease characterized by persistent airflow obstruction
and abnormalities of the airways (bronchitis/bronchiolitis) and/or alveoli (emphysema) leading to2:
Symptoms (dyspnea, sputum production, cough)
Exacerbations
6th leading cause of death in the US in 20211
Frequent exacerbations and hospitalizations drive the cost of COPD-related healthcare in the US, which is projected to rise3-4*
$31.3 billion (2019) → $60.5 billion (2029)
*COPD-attributable expenditure estimates for 2019 were generated based on cross-sectional, retrospective study of 4135 people with COPD using the Medical Expenditure Panel Survey (2016-2019), the American Community Survey (2019), and Behavioral Risk Factor Surveillance System data (2019). Cost projections were based on population projections reported by the US Census Bureau (2017) and adjusted to the 2019 US dollar.3
References: 1. Liu Y, et al. MMWR Morb Mortal Wkly Rep. 2023;72:1250-1256. 2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024 Report. www.goldcopd.org. Accessed September 19, 2024. 3. Mannino D, et al. Chest. 2024;165(5):1093-1106. 4. Larsen DL, et al. Am Health Drug Benefits. 2022;15(2):57-64.
DR. CRINER:
COPD is a progressive inflammatory airway disease that affects more than 14 million Americans. It is characterized by persistent airflow obstruction and abnormalities of the airway and alveoli.
Many of our patients complain of symptoms like dyspnea, sputum production, and cough.
Patients also frequently experience exacerbations, which have a negative impact on their health-related quality of life and COPD prognosis.
Exacerbations are the primary reason why COPD remains the sixth leading cause of death in the United States.
Exacerbations are also a major driver of COPD-related costs in the US, which are only expected to rise in the coming years. By 2029, COPD is projected to cost our healthcare system an estimated 60 billion dollars.
As a clinician, it’s an important priority for me to identify those patients at a greater risk of exacerbations. This is critical to help them manage their disease effectively and prevent COPD worsening as much as possible.
ONSCREEN TEXT:
COPD Exacerbations Are Key Drivers of Disease Progression That Place Patients at Increased Risk of Further Exacerbations and Death1-5
The strongest predictor of future exacerbation risk is a prior history of exacerbations1,2
Median Inter-Exacerbation Times Following First COPD Hospitalization3,*
[Line graph showing the time after first severe exacerbation (years) on the x-axis and the rate of next severe exacerbation per 10,000 per day on the y-axis. The figure shows the median time between successive severe exacerbations decreases with every new severe exacerbation.]
Reprinted from Suissa S, et al. Thorax. 2012;67(11):957-963. Copyright © 2012, The Author(s). Published by BMJ Publishing Group Ltd.
Risk in Study of Medicare Beneficiaries†
13.7x higher incidence of hospitalization
12.6x higher incidence of death
within the first 30 days of discharge for COPD-related hospitalization vs general population of Medicare beneficiaries4
*Retrospective cohort study using healthcare databases from Canada that analyzed time of first ever hospitalization for COPD (1990-2005) to death or March 2007, over a 17-year follow-up period (N=73,106).3
†Study to analyze risk of readmission and death after discharge in Medicare beneficiaries aged ≥65 years hospitalized with COPD (2008-2014; principal diagnosis of COPD or acute respiratory failure with a secondary diagnosis of COPD with acute exacerbation, per ICD-9-CM). Readmission was evaluated in 2,340,637 patients and death in 1,283,069 patients. Comparator population (N=23,052,575) comprised of 2012 Medicare beneficiaries aged ≥65 years on December 31, 2011, with ≥12 months of enrollment.4
ICD-9-CM=International Classification of Diseases, Ninth Revision, Clinical Modification.
References: 1. Hurst JR, et al. N Engl J Med. 2010;363(12):1128-1138. 2. Hurst JR et al. Am J Respir Crit Care Med. 2009;179:369-374. 3. Suissa S, et al. Thorax. 2012;67:957-963. 4. Lindenauer PK, et al. Am J Respir Crit Care Med. 2018;197(8):1009-1017. 5. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024 Report. www.goldcopd.org.
Accessed September 19, 2024.
DR. CRINER:
Each COPD exacerbation puts patients at an increased risk of future exacerbations and death. Patients can get stuck in a cycle of exacerbations leading to progressive disease worsening and decline.
For patients with COPD, the best indicator of exacerbation risk is a prior exacerbation. We can see this clearly in the data shown on the left.
This study included more than 73,000 Canadian patients with COPD, hospitalized for their first severe exacerbation. We can see that following the first event, the time between subsequent exacerbations decreased from one event to the next.
On the right, we’re looking at a study of 2.3 million Medicare beneficiaries. Patients hospitalized for a COPD exacerbation had a 13.7 times greater risk of re-hospitalization and 12.6 times greater risk of death within 30 days of hospital discharge compared with a general Medicare population.
Together, these data demonstrate just how impactful COPD exacerbations can be on patient mortality.
Minimizing hospitalizations is a key priority for me, as we know that hospitals can present further morbidity risks for our patients, including development of infections like pneumonia.
ONSCREEN TEXT:
About Half of Patients With COPD and a History of Exacerbations Continued to Experience Exacerbations, Despite Maximized Inhaled Therapy
~50% of patients on triple therapy experienced ≥1 exacerbation in the first year*,†
*Phase 3, 52-week, randomized, double-blind, parallel-group, multicenter trial. The primary objective was to evaluate the effects of 52 weeks of two doses of ICS/LAMA/LABA therapy compared with ICS/LABA or LAMA/LABA therapy on the rate of moderate or severe COPD exacerbations. Moderate exacerbations were defined as those leading to treatment with systemic glucocorticoids, antibiotics, or both for at least 3 days; severe exacerbations were defined as those resulting in hospitalization or death. The exacerbation rate was 49.8% for patients on LAMA/LABA (n=1056/2120), 50.9% for patients on ICS/LABA (n=1085/2131), and 48.0% for patients on ICS/LABA/LAMA (n=1026/2137).
†Based on time to first moderate or severe COPD exacerbation over 52 weeks (secondary endpoint).
ICS=inhaled corticosteroid; LABA=long-acting beta2-adrenergic agonist; LAMA=long-acting muscarinic antagonist.
Reference: Rabe KF, et al. N Engl J Med. 2020;383(1):35-48.
DR. CRINER:
Despite appropriate management with inhaled maintenance therapies, we know that many patients continue to exacerbate.
GOLD recommends triple therapy for patients who continue to exacerbate. However, even for patients who have maximized their inhaled therapy with an ICS/LABA/LAMA, there still is a need for exacerbation reduction.
These data are from a 52-week study evaluating inhaled maintenance treatments for exacerbation reduction in patients with COPD who had a history of recent exacerbations. Patients were randomized to receive either a dual combination of LABA/LAMA, a combination of inhaled corticosteroid and a LABA, or triple therapy with an inhaled corticosteroid and dual long-acting bronchodilators.
You can see that about 50% of patients receiving triple therapy still experienced at least one exacerbation in that year. This was also seen in patients receiving LAMA/LABA and ICS/LABA.
ONSCREEN TEXT:
OCS Are Widely Used to Help Manage Exacerbations, but Cumulative Exposure Is Associated With Increased Risk of Adverse Health Outcomes1,2
In a retrospective claims study of patients with COPD1,*:
Higher incidence of new conditions/events with OCS >1000 mg vs no OCS use within 48 months after diagnosis, including
CV disease
Heart failure
Hypertension
Obesity
Dyspepsia
Infections
Depression/anxiety
Higher risk of adverse health outcomes associated with greater OCS exposure
In a retrospective claims study of COPD patients who received ≥1 course of OCS, in the 12 months following triple therapy initiation (N=2013)2:
[Pie chart showing 67.8% shaded in orange] 67.8% of patients had ≥1 OCS claim, with a mean of 2.5 courses per patient†
*Medicare administrative data claim study (N=183,637): patients aged ≥40 years with a new diagnosis of COPD per ICD-9-CM identified from ≥1 acute or non-acute inpatient, ED, or observation claim in 2015. Study excluded patients with diagnoses of conditions commonly treated with OCS in the baseline period or 12 months pre-index date and patients who developed any exclusionary condition >12 months post-index date. Patients were followed up for 12 to 48 months post-index. Analysis of patients with 48 months of data included 6983 receiving OCS >1000 mg (prednisolone equivalent) and 118,112 with no OCS use.1
†Compared with mean of 3.3 OCS courses in the 12-month pre-index period.2
CV=cardiovascular; ED=emergency department; OCS=oral corticosteroid.
References: 1. Bazell C, et al. Int J Chron Obstruct Pulmon Dis. 2022;17:2635-2652. 2. Bogart M, et al. Int J Chron Obstruct Pulmon Dis. 2023;18:2367-2379.
DR. CRINER:
While oral corticosteroids remain an invaluable tool to help manage COPD exacerbations, cumulative exposure to OCS places our patients at an increased risk of complications.
The data on the left are from a claims-based analysis of almost 7000 patients with COPD who received more than 1000 milligrams of oral corticosteroids in the 48 months following their COPD diagnosis.
These patients had a higher incidence of new conditions, like those listed here, compared with patients not receiving OCS. These risks increased with greater steroid exposure. I’m particularly concerned about effects like cardiovascular disease and related complications. This is especially true for my patients with COPD who already have comorbidities like these that may be worsened by OCS use.
Despite these risks, OCS use remains widespread. The data on the right are from a study of over 2000 patients who received at least 1 course of OCS within 12 months of initiating inhaled triple therapy.
During that time, nearly 70% of patients received at least 1 course of OCS with an average of 2 and a half courses per patient. This tells us that, even though maximum inhaled therapy can help reduce OCS use for many, it remains high for some patients.
It's clear to me that reducing OCS use continues to be an unmet need in COPD management, and as clinicians, we should be selective about when we prescribe them. GOLD recommends limiting OCS courses and only using them to treat patients with significant exacerbations. This includes reducing the need for OCS by preventing exacerbations.
ONSCREEN TEXT:
COPD Exacerbations Negatively Impact Health-Related Quality of Life, Which Can Further Increase Exacerbation Risk1-7
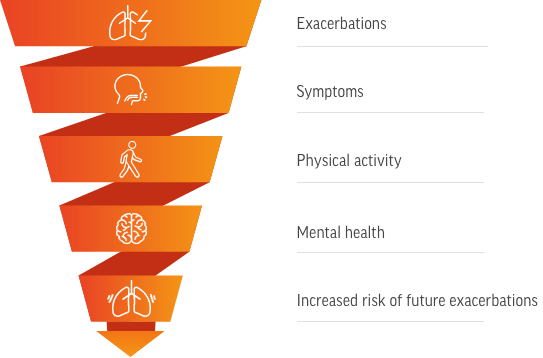
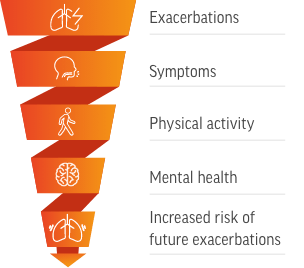
[Downward spiral graphic with the following labels from top to bottom: Exacerbations, Symptoms, Physical activity, Mental health, Increased risk of future exacerbations]
Worsening health-related quality of life increases the risk of future exacerbations, contributing to the cycle of disease worsening1
References: 1. Hurst JR, et al. Eur J Intern Med. 2020;73:1-6. 2. Seemungal TAR, et al. Am J Respir Crit Care Med. 1998;157:1418-1422. 3. Guo J, et al. Int J Surgery. 2020;22:28-35. 4 St George’s University of London SGRQ manual. Accessed October 1, 2024. https://www.sgul.ac.uk/research/research-operations/research-administration/st-georges-respiratory-questionnaire/docs/SGRQ-Manual-March-2022.pdf. 5. Atlantis E, et al. Chest. 2013;144(3):766-777. 6. Eslaminejad A, et al. J Health Psychol. 2017;22(12):1603-1613. 7. Spina G, et al. Thorax. 2017;72(8):694-701.
DR. CRINER:
I’m also concerned about the impact of COPD exacerbations on my patient’s quality of life. As health-related quality of life declines, the risk of future exacerbations increases.
Some key aspects of quality of life that can be impacted by an exacerbation include symptoms, physical activity, and mental health.
These components of patient wellbeing are interrelated, and impairment of one can impact the others. This compounding effect can further reduce a patient’s overall quality of life.
For example, just one exacerbation can lead to a significant decline in lung function, which can result in greater symptom burden and physical impairments. Symptoms can impact a patient throughout the whole day, limiting their ability to perform daily activities, reducing exercise capacity, and disrupting sleep.
Sleep disturbances contribute to fatigue, which is a common symptom of COPD. Sleep can be impacted by several health-related factors, like difficulty breathing and anxiety. In turn, impairments on a patient’s ability to rest can further exacerbate their physical limitations and contribute to worse mental health.
Mental health issues, like anxiety and depression, are more common among patients with COPD compared with the general population. And our patients with frequent exacerbations are at an even greater risk of these comorbidities. Anxiety and depression can then further contribute to reduced physical activity and worsening quality of life.
Most importantly, this overall worsening of health-related quality of life places patients at an increased risk of exacerbations, reinforcing the cycle of disease worsening.
Coupled with the unpredictability of future exacerbations, these issues make it more difficult for patients with COPD to plan for the activities in their life that are most important to them and may contribute to them ultimately becoming housebound.
ONSCREEN TEXT:
What is the emerging science on type 2 inflammation in respiratory diseases?
DR. CRINER:
Despite our best efforts, we’ve all had patients who continue to exacerbate on maximum inhaled therapy. It leaves us as clinicians wondering what more we can do to improve patient outcomes.
Understanding the mechanisms driving COPD is a key part of improving patient care.
Many of us are familiar with type 2 inflammation in the context of asthma, but the latest evidence suggests that type 2 inflammation may play an important role in some patients with COPD as well.
So, it’s important for us to understand how type 2 inflammation can contribute to pathophysiological processes in airway diseases.
ONSCREEN TEXT:
Type 2 Inflammation in Airway Diseases Is Driven by Key Inflammatory Cytokines1-4
IL-5, IL-4, and IL-13 are key cytokines that contribute to type 2 inflammation through1-5:
Eosinophil recruitment and trafficking
Type 2 inflammatory cell activation
Perpetuation of type 2 inflammatory cascade and its consequences
IL-5 plays an important role in type 2 inflammation as the predominant mediator of eosinophil activation, differentiation, proliferation, and survival1-6
[Images depicting IL-5, IL-4, and IL-13 above images depicting Eosinophil, ILC2, T cell, Mast cell, Macrophage, and Plasma/B cell]
IL=interleukin; ILC2=group 2 innate lymphoid cell.
References: 1. Buchheit KM, et al. Allergy. 2024;79(10):2662-2679. 2. Maspero J, et al. ERJ Open Res. 2022;8(3):00576-2021. 3. Pelaia C, et al. Front Pharmacol. 2022;13:851940. 4. Rabe KF, et al. Am J Respir Crit Care Med. 2023;208(4):395-405. 5. Brightling C, et al. Eur Respir J. 2019;54(2):1900651. 6. Bafadhel M, et al. Respiration. 2009;78(3):256-262.
DR. CRINER:
Type 2 inflammation is driven by cytokines including interleukin-5, interleukin-4, and interleukin-13. These cytokines contribute to inflammation by recruiting and activating several inflammatory cell types, like those shown here.
The hallmark feature of type 2 inflammation is the presence of elevated blood eosinophils.
As you know, eosinophils are a type of white blood cell that contribute to innate and adaptive immunity and are commonly associated with defense against parasitic infections. However, elevated levels of activated eosinophils are associated with several type 2 inflammatory diseases.
Eosinophils contribute to type 2 inflammation through the release of pro-inflammatory mediators, including interleukin-5, interleukin-4, and interleukin-13.
Evolving evidence indicates that interleukins 5, 4, and 13 can impact airway structural cells, as we will discuss over the next few minutes.
Interleukin-5 has an important role in mediating type 2 inflammation because it is the predominant cytokine responsible for the activation, differentiation, proliferation, and survival of eosinophils.
Beyond its effect on eosinophils, emerging evidence also supports a role for interleukin-5 in modulating immune function by targeting other inflammatory cells. This includes group 2 innate lymphoid cells, also known as ILC2 cells, T cells, mast cells, and plasma cells.
ONSCREEN TEXT:
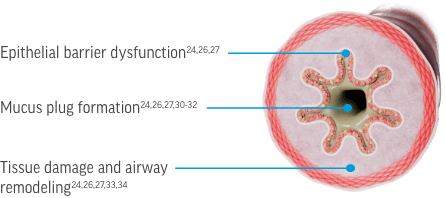
IL-5, IL-4, and IL-13 Contribute to Airway Pathophysiology Through Interactions With a Broad Network of Immune and Structural Cells1-5
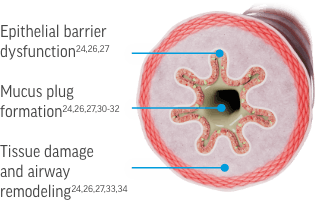
[Figure of airway cross section with epithelial cells highlighted]
Epithelial barrier dysfunction1-3,6-9 [Text appears on an arrow pointing to a box with images of the key cytokines and cells involved. Next to this text on the airway cross section are 2 grayed-out labels: “Mucus plug formation” and “Tissue damage and airway remodeling”]
Key cytokines and cells involved
IL-5
IL-4
IL-13
Eosinophil
Mast cell
Epithelial cell
Fibroblast
References: 1. Buchheit KM, et al. Allergy. 2024;79(10):2662-2679. 2. Maspero J, et al. ERJ Open Res. 2022;8(3):00576-2021. 3. Pelaia C, et al. Front Pharmacol. 2022;13:851940. 4. Rabe KF, et al. Am J Respir Crit Care Med. 2023;208(4):395-405. 5. Brightling C, et al. Eur Respir J. 2019;54(2):1900651. 6. Barretto KT, et al. Allergy. 2020;75:2127-2130. 7. Saatian B, et al. Tissue Barriers. 2013;1(2):e24333. 8. Jacobsen EA, et al. Annu Rev Immunol. 2021;39:719–757. 9. Higham A, et al. J Leukoc Biol. 2024;116(5):927-946.
DR. CRINER:
In the context of respiratory disease, type 2 inflammation can impact the airway through several mechanisms. The science is still evolving, but we are now beginning to understand the role of type 2 inflammation in epithelial barrier dysfunction, mucus plug formation, tissue damage and airway remodeling.
Recent evidence has begun to identify the cells and cytokines most closely involved in driving these processes in airway diseases. I think it’s important for us to talk in a bit more detail about each process and the cells involved in the pathology described.
In the healthy airway, ciliated epithelial cells are the first line of defense against pathogens and irritants. They form a dense barrier reinforced by tight junction proteins.
Epithelial cells also produce inflammatory signaling molecules that activate and recruit the immune cells needed to fight infection.
In airway diseases like COPD, chronic epithelial injury and inflammation lead to a defective epithelial barrier.
Interleukins 5, 4, and 13 contribute to the downregulation of tight-junction proteins and disrupt replacement of damaged epithelial cells.
This results in a fragmented epithelial barrier that is more vulnerable to bacterial and viral infections.
Eosinophils, mast cells, and fibroblasts can also play a role in regulating epithelial barrier function. Targeting of these cells by interleukins 5, 4, and 13 can contribute to tissue fibrosis, effects on the basement membrane, and collagen production.
ONSCREEN TEXT:
IL-5, IL-4, and IL-13 Contribute to Airway Pathophysiology Through Interactions With a Broad Network of Immune and Structural Cells1-5
[Figure of airway cross section with mucus highlighted]
Mucus plug formation1-3,6-8 [Text appears on an arrow pointing to a box with images of the key cytokines and cells involved. Next to this text on the airway cross section are 2 grayed-out labels: “Epithelial barrier dysfunction” and “Tissue damage and airway remodeling”]
Key cytokines and cells involved
IL-5
IL-4
IL-13
Eosinophil
Epithelial cell
Goblet cell
References: 1. Buchheit KM, et al. Allergy. 2024;79(10):2662-2679. 2. Maspero J, et al. ERJ Open Res. 2022;8(3):00576-2021. 3. Pelaia C, et al. Front Pharmacol. 2022;13:851940. 4. Rabe KF, et al. Am J Respir Crit Care Med. 2023;208(4):395-405. 5. Brightling C, et al. Eur Respir J. 2019;54(2):1900651. 6. Dunican EM, et al. J Clin Invest. 2018;128:997–1009. 7. Echevarría L, et al. Int J Chron Obstruct Pulmon Dis. 2017;12:885-896. 8. Persson EK, et al. Science. 2019;364:751.
DR. CRINER:
Type 2 cytokines can also drive mucus plug formation.
Normally, mucus protects the airway from irritation and infection by trapping and removing debris and pathogens.
However, mucus that is too viscous, abundant, or immobile can accumulate in the airways as mucus plugs. Mucus plugs can obstruct the airway and are associated with an increased risk of death.
Mucus dysfunction also contributes to sputum production, cough, and dyspnea, and leaves patients more susceptible to inflammation and infection.
All three cytokines contribute to the loss of ciliated epithelial cells which normally propel mucus and trapped pathogens out of the airways.
Interleukin-5 further drives mucus plug development by increasing mucus production and viscosity. This occurs through the formation of eosinophilic DNA traps and Charcot-Leyden crystals in the airway.
Interleukin-13 can increase mucus production by increasing goblet cell numbers.
ONSCREEN TEXT:
IL-5, IL-4, and IL-13 Contribute to Airway Pathophysiology Through Interactions With a Broad Network of Immune and Structural Cells1-5
[Figure of airway cross section with airway tissue highlighted]
Tissue damage and airway remodeling1-3,6-9 [Text appears on an arrow pointing to a box with images of the key cytokines and cells involved. Next to this text on the airway cross section are 2 grayed-out labels: “Epithelial barrier dysfunction” and “Mucus plug formation”]
Key cytokines and cells involved
IL-5
IL-4
IL-13
Eosinophil
Macrophage
Mast cell
Epithelial cell
Fibroblast
Smooth muscle
References: 1. Buchheit KM, et al. Allergy. 2024;79(10):2662-2679. 2. Maspero J, et al. ERJ Open Res. 2022;8(3):00576-2021. 3. Pelaia C, et al. Front Pharmacol. 2022;13:851940. 4. Rabe KF, et al. Am J Respir Crit Care Med. 2023;208(4):395-405. 5. Brightling C, et al. Eur Respir J. 2019;54(2):1900651. 6. Siddiqui S, et al. J Allergy Clin Immunol. 2023;152:841-857. 7. Doyle AD, et al. Eur Respir J. 2019;53(5):1801291. 8. Bajbouj K, et al. Allergy. 2023;78(3):882-885. 9. Higham A, et al. J Leukoc Biol. 2024;116(5):927-946.
DR. CRINER:
Type 2 inflammation drives repeated cycles of tissue destruction and repair that can result in airway remodeling, which can reduce airway elasticity and impair gas exchange.
Tissue destruction can occur throughout the airway, but it is particularly problematic in the alveoli. Breakdown of the alveolar walls reduces the surface area available for gas exchange. This results in diminished oxygen supply in the blood.
Conversely, fibrosis is an example of an exaggerated repair response. This is driven by an overproduction of extracellular matrix proteins like collagen, creating thick and rigid scar tissue that impairs the flexing and recoil needed for exhalation.
Interleukins 5, 4, and 13 promote transformation of epithelial cells into collagen-secreting cells and fibroblast proliferation, leading to fibrosis and airway rigidity.
Interleukin-5 can also contribute to airway remodeling via eosinophils, which release cytokines, proteases, and other substances like TGF-beta, a key mediator of airway remodeling.
Interleukins 4 and 13 can promote tissue degradation by macrophages.
Lastly, interleukins 5 and 13 have been linked to airway smooth muscle hypertrophy.
Collectively, these effects can lead to fibrosis, airway wall thickening, and airway rigidity.
ONSCREEN TEXT:
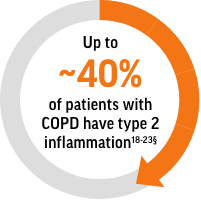
Type 2 Inflammation, Characterized by Blood Eosinophils, Is Common in Patients With COPD1-6,*
Up to ~40% of patients with COPD have type 2 inflammation*
Blood eosinophils are an established biomarker for type 2 inflammation in patients with COPD7-10
*As determined by blood eosinophil count.
References: 1. Tashkin D, Wechsler M. Int J Chron Obstruct Pulmon Dis. 2018;13:335-349. 2. Singh D, et al. Eur Respir J. 2014;44(6):1697-1700. 3. Trudo F, et al. Int J Chron Obstruct Pulmon Dis. 2019;14:2625–2637. 4. Landis SH, et al. Respir Med. 2019;X(1):100011. 5. Oshagbemi OA, et al. Am J Respir Crit Care Med. 2017;195(10):1402-1404. 6. David B, et al. Thorax. 2021;76:188–195. 7. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024 Report. www.goldcopd.org. Accessed September 19, 2024. 8. Rabe KF, et al. Am J Respir Crit Care Med. 2023;208(4):35-48. 9. Singh D, et al. Am J Respir Crit Care Med. 2022;206(1):17-24. 10. Maspero J, et al. ERJ Open Res. 2022;8(3):00576-2021.
DR. CRINER:
Historically, it was believed that COPD was driven solely by neutrophilic inflammation. However, it’s now known that up to approximately 40% of patients have type 2 inflammation as identified by blood eosinophils.
Elevated blood eosinophils are the biomarker that I look for when identifying which of my patients with COPD has underlying type 2 inflammation.
ONSCREEN TEXT:
Airway Inflammation Contributes to Clinical Manifestations of COPD1
Per GOLD, chronic airway inflammation has been implicated in the structural changes that contribute to clinical manifestations of COPD including1
Exacerbations
Symptoms (shortness of breath, cough, sputum production)
Irreversible airflow limitation
The role of type 2 inflammation in COPD is an area of evolving research2,3
References: 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024 Report. www.goldcopd.org. Accessed September 19, 2024. 2. Brightling C, et al. Eur Respir J. 2019;54(2):1900651. 3. Higham A, et al. J Leukoc Biol. 2024;116(5):927-946.
DR. CRINER:
While our understanding into type 2 inflammation and its consequences in COPD is still evolving, we know that chronic exposure to pollutants, like cigarette smoke, results in inflammation within the airway for all patients with COPD.
This chronic inflammation drives structural changes that contribute to the clinical manifestations that we see every day in our COPD patients. These include exacerbations, symptoms such as dyspnea, and irreversible airflow limitation.
ONSCREEN TEXT:
How can we use blood eosinophils as a biomarker to identify patients with type 2 inflammation?
DR. CRINER:
So now, I’d like to talk about how and why we should use blood eosinophil count to identify patients with COPD with underlying type 2 inflammation.
ONSCREEN TEXT:
Increased Blood Eosinophil Count Is Associated With More Exacerbations and COPD-Related ED Visits or Hospitalizations
Patients with a baseline blood eosinophil count ≥300 cells/µL experienced significantly more COPD exacerbations vs patients with lower counts*
[Bar chart depicting the rate ratio of COPD exacerbations occurring by baseline blood eosinophil count:
≥50 vs <50 cells/μL, 0.97
≥150 vs <150 cells/μL, 1.09
≥300 vs <300 cells/μL, 1.25† (highlighted in orange)
≥400 vs <400 cells/μL, 1.48† (highlighted in orange)
≥500 vs <500 cells/μL, 1.76† (highlighted in orange)]
Rates of COPD-related ED visits or hospitalizations were significantly greater for patients with a baseline blood eosinophil count ≥400 cells/µL*
[Bar chart depicting the rate ratio of COPD-related ED visits or hospitalizations occurring by baseline blood eosinophil count:
≥50 vs <50 cells/μL, 0.9
≥150 vs <150 cells/μL, 0.98
≥300 vs <300 cells/μL, 1.18
≥400 vs <400 cells/μL, 1.44‡ (highlighted in blue)
≥500 vs <500 cells/μL, 1.68† (highlighted in blue)]
*Retrospective cohort study using Kaiser Permanente Southern California Research Data Warehouse that examined the relationship between blood eosinophil count and COPD exacerbations in the one-year follow-up in 7245 patients with COPD between 2009 and 2012.
†P<0.001.
‡P=0.002.
Reference: Zeiger RS, et al. J Allergy Clin Immunol Pract. 2018;6:944-954.
DR. CRINER:
Blood eosinophil counts can help predict patients at a higher risk of COPD exacerbations and hospitalizations.
These data are from a retrospective study assessing COPD exacerbations in the year following a blood eosinophil measurement.
Patients were distributed across prespecified blood eosinophil thresholds ranging from 50 to greater than or equal to 500 cells per microliter.
On the left, you can see that each bar represents the rate ratio of exacerbations in patients with a blood eosinophil count falling above the threshold compared with those below the threshold.
There is a clear trend showing that patients with higher blood eosinophil counts had more exacerbations.
These comparisons reached significance with the 300 cells per microliter group, as indicated by the orange highlight, and continued to hold true for groups with increasing blood eosinophils.
Ultimately, what this means is that patients with at least 300 cells per microliter had 25% more exacerbations compared with patients below that threshold. The rate of exacerbations went up by 48% for the 400 cells per microliter group and by 76% for the 500 cells per microliter group compared to patients below those thresholds, respectively.
These data show how elevated blood eosinophil counts can be predictive of exacerbation frequency.
On the right, you see a similar trend showing more emergency department visits and hospitalizations with higher blood eosinophils. Here, significance was reached in patients with blood eosinophils at or above 400 cells per microliter, where patients had 44% more hospitalizations and emergency department visits compared to those with blood eosinophil counts below 400 cells per microliter. Patients had 68% more of these visits if their blood eosinophil count was greater than 500 cells per microliter compared to patients below this threshold.
Together these data illustrate that patients with higher blood eosinophils have a greater risk of future exacerbations, emergency department visits, or hospitalizations, highlighting the value of blood eosinophils as a predictive biomarker in patients with COPD.
ONSCREEN TEXT:
The GOLD Report Supports Evaluating Blood Eosinophils as a Treatable Trait to Support COPD Management1
Treatable traits are patient characteristics that can be assessed to guide management1,2
Examples of phenotypic traits
Symptoms
Exacerbations
Example of endotypic trait
Blood eosinophil count
Evaluated in CBC with differential3
Per GOLD, treatable traits can co-exist in a patient and change with time1
CBC=complete blood count.
References: 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024 Report. www.goldcopd.org. Accessed September 19, 2024. 2. Thomas M, et al. Respirology. 2023;28(12):1101-1116. 3. Tefferi A, et al. Mayo Clin Proc. 2005;80(7):923-936.
DR. CRINER:
According to GOLD, blood eosinophil count can be evaluated as a treatable trait that can help guide COPD management.
We’re all familiar with the treatable traits paradigm published by GOLD for patient evaluation and management. This framework was intended to help address the heterogeneity and complexity of COPD presentation that we see in clinical practice. It recommends a few key patient characteristics that can be routinely evaluated. These include phenotypic traits, like symptoms and exacerbations that we can observe clinically, and endotypic traits that rely on a deeper understanding of the pathways underlying COPD.
GOLD mentions the endotype of elevated blood eosinophils, which are established as a biomarker that can predict the effectiveness of treatment with an inhaled corticosteroid.
It’s worth noting that all of these features—like symptoms, exacerbations, and blood eosinophils—should be taken into consideration collectively to help guide management decisions.
The good news is that you can routinely measure blood eosinophils in your patients with COPD; this information is often readily available.
Since blood eosinophil count is measured as a component of a standard CBC with differential, historical counts may already be recorded in the patient’s medical record. I think it’s worth a quick search to find that information.
ONSCREEN TEXT:
Blood Eosinophil Count Is an Easy-to-Obtain Indicator of Type 2 Inflammation1-5
Blood Eosinophil Counts in COPD Management Considerations
Between-Treatment Ratios for Rates of Moderate or Severe Exacerbations2,*
[Line graph depicting exacerbation rate ratios (95% CI) as a function of baseline blood eosinophil count (eosinophils per µL) in patients who received ICS/LAMA/LABA vs LAMA/LABA (blue line) and ICS/LABA vs LAMA/LABA (orange line). A horizontal dotted line is included at 1.0. The exacerbation rate ratios for both comparisons decrease as baseline blood eosinophil count increases.]
Reprinted from Pascoe S, et al. Lancet Respir Med. 2019;7(9):745-756. Copyright © 2019 Elsevier Ltd.
Per GOLD, when added to maintenance bronchodilator therapy1:
<100 eosinophils/μL
Patients unlikely to benefit from ICS
≥300 eosinophils/μL
Patients with greatest likelihood to benefit from ICS
Blood eosinophil counts should not be interpreted in isolation when making individual patient treatment decisions in COPD3
*An analysis of the IMPACT study that modelled the relationship of blood eosinophil counts with ICS therapy on exacerbations in 10,333 patients with COPD.2
CI=confidence interval.
References: 1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024 Report. www.goldcopd.org. Accessed September 19, 2024. 2. Pascoe S, et al. Lancet Respir Med. 2019;7(9):745-756. 3. Pascoe S, et al. Lancet Respir Med. 2018;6(5):e18. 4. Tefferi A, et al. Mayo Clin Proc. 2005;80(7):923-936. 5. David B, et al. Thorax. 2021;76(2):188-195.
DR. CRINER:
The GOLD report states that blood eosinophils can be used to guide the use of ICS for patients with COPD as part of pharmacological management.
These treatment recommendations were based on clinical evidence showing that increasing blood eosinophil counts correlate with greater efficacy of ICS at preventing future exacerbations.
This study included more than 10,000 patients with COPD and a history of exacerbation in the previous year. Patients were randomized to receive either a LAMA/LABA, an ICS/LABA, or triple therapy.
What we see in the figure is a continuous relationship between ICS treatment effect for reducing moderate and severe exacerbations, shown on the y-axis at the left as rate ratio, and increasing eosinophil count, shown on the x-axis at the bottom. These data show that ICS is better at reducing exacerbations at higher blood eosinophil counts.
Let’s break this down in a bit more detail.
The orange line depicts the comparison between ICS/LABA and LAMA/LABA, and the blue line is the comparison between triple therapy and LAMA/LABA. The rate ratio indicates how well the ICS containing therapies reduced exacerbations compared with LAMA/LABA. Lower rate ratios indicate a greater exacerbation reduction with the ICS-containing therapies. When the orange and blue lines are at or above 1, as shown on the left side of the figure for patients with lower eosinophil counts, we can conclude that the addition of ICS had little or no effect. Conversely, on the right side of the figure, for patients with higher eosinophil counts, both lines drop below the rate ratio 1, indicating that the ICS-containing medications reduce exacerbations compared with LAMA/LABA.
Overall, you can see that as the eosinophil count increases so does the exacerbation reduction benefit we see with the ICS-containing treatments compared with LAMA/LABA.
What this means for us in the clinic is that we can and should be using blood eosinophil count to identify which patients are most likely to benefit from an ICS.
According to GOLD, and as we can clearly see in these data, patients with blood eosinophil counts less than 100 cells per microliter are unlikely to benefit from ICS-containing therapies. Conversely, a blood eosinophil count of at least 300 cells per microliter indicates those patients with the greatest likelihood of treatment benefit with ICS.
These data reinforce the importance of including eosinophil count along with other clinical characteristics when we are making treatment decisions for our patients.
ONSCREEN TEXT:
Summary of Key Insights on Type 2 Inflammation and the Role of Eosinophils in COPD
Type 2 inflammation characterized by blood eosinophils in patients with COPD is associated with increased risk, frequency, and severity of exacerbations1,2
Key type 2 inflammatory cytokines, eg, IL-5, IL-4, and IL-13, play an important role in type 2 inflammation3-8
Check your patients’ blood eosinophil count to identify underlying type 2 inflammation:
Blood eosinophils are an established, easy-to-obtain clinical biomarker to identify patients with underlying type 2 inflammation, and help guide COPD management according to the GOLD Report9-14
References: 1. Zeiger RS, et al. J Allergy Clin Immunol Pract. 2018;6:944-954. 2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024 Report. www.goldcopd.org. Accessed September 19, 2024. 3. Rabe KF, et al. Am J Respir Crit Care Med. 2023;208(4):395-405. 4. Maspero J, et al. ERJ Open Res. 2022;8(3):00576-2021. 5. Brightling C, et al. Eur Respir J. 2019;54(2):1900651. 6. Higham A, et al. J Leukoc Biol. 2024;116(5):927-946. 7. Buchheit KM, et al. Allergy. 2024;79(10):2662-2679. 8. Pelaia C, et al. Front Pharmacol. 2022;13:851940. 9. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2024 Report. www.goldcopd.org. Accessed September 19, 2024. 10. Thomas M, et al. Respirology. 2023;28(12):1101-1116. 11. Tefferi A, et al. Mayo Clin Proc. 2005;80(7):923-936. 12. Pascoe S, et al. Lancet Respir Med. 2019;7(9):745-756. 13. Pascoe S, et al. Lancet Respir Med. 2018;6(5):e18. 14. David B, et al. Thorax. 2021;76(2):188-195.
DR. CRINER:
As we conclude, let’s recap the key points we covered in this program.
We now know that type 2 inflammation, as measured by blood eosinophil count, is present in many patients with COPD and is associated with a greater risk of exacerbations.
These exacerbations are critical events in COPD progression and can lead to significant negative impacts on our patients’ overall health and wellbeing.
Interleukins 5, 4 and 13 are key cytokines that play an important role in type 2 inflammation. As the science continues to evolve, a key goal is to better understand how type 2 inflammation impacts the airway in patients with COPD. These insights may help identify COPD patients at risk of exacerbations.
If you take one thing away from this discussion, it would be the importance of checking the blood eosinophil level in your patients with COPD. Blood eosinophils are an established, easy-to-obtain clinical biomarker. It can help identify those patients with underlying type 2 inflammation, including those at elevated risk of exacerbations, and give you some insight into how to better manage their care.
ONSCREEN TEXT:
Thank You
Trademarks are owned by or licensed to the GSK group of companies.
[GSK logo]
©2024 GSK or licensor.
NPUS-CPUSTBD240002 November 2024
Produced in USA.