Our eosinophilosophy
eos counts in
EOSINOPHILIC GRANULOMATOSIS
WITH POLYANGIITIS
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome, is a disorder classified as a small-vessel vasculitis, and is associated with eosinophilia and asthma. EGPA is characterized by eosinophil-rich inflammation and necrotizing granulomatous inflammation that often affects the respiratory tract, and necrotizing vasculitis that often affects small- to medium-sized blood vessels.1-3 Effects of the inflammation can lead to clinical manifestations that can vary across patients.3,4
EGPA is a rare disorder. Estimated prevalence of EGPA is 5000 patients in the US.5,6
EGPA is a 3-phase disorder4,7
Not all patients experience all phases. Phases do not have to follow one another and may overlap.
Prodromal phase:
Asthma, typically adult onset, rhinosinusitis, and nasal polyposis. Asthma is the main manifestation and it may precede other manifestations by many years
Eosinophilic phase:
Elevated eosinophils in the blood and/or tissue
Vasculitic phase:
Inflammation of the blood vessels that can reduce blood flow to organs
Eosinophilic inflammation is a key feature in EGPA
Activated eosinophils have proinflammatory effects on tissue they have infiltrated through the release of lipid mediators and cytotoxic granule proteins. These proinflammatory effects lead to tissue damage. In the eosinophilic phase, the lungs, heart, and gastrointestinal system are often affected. Eosinophils also infiltrate the blood vessels during the vasculitic phase. Organs affected during this phase depend on which blood vessels are affected.7
Eosinophilia in EGPA pathogenesis7-9
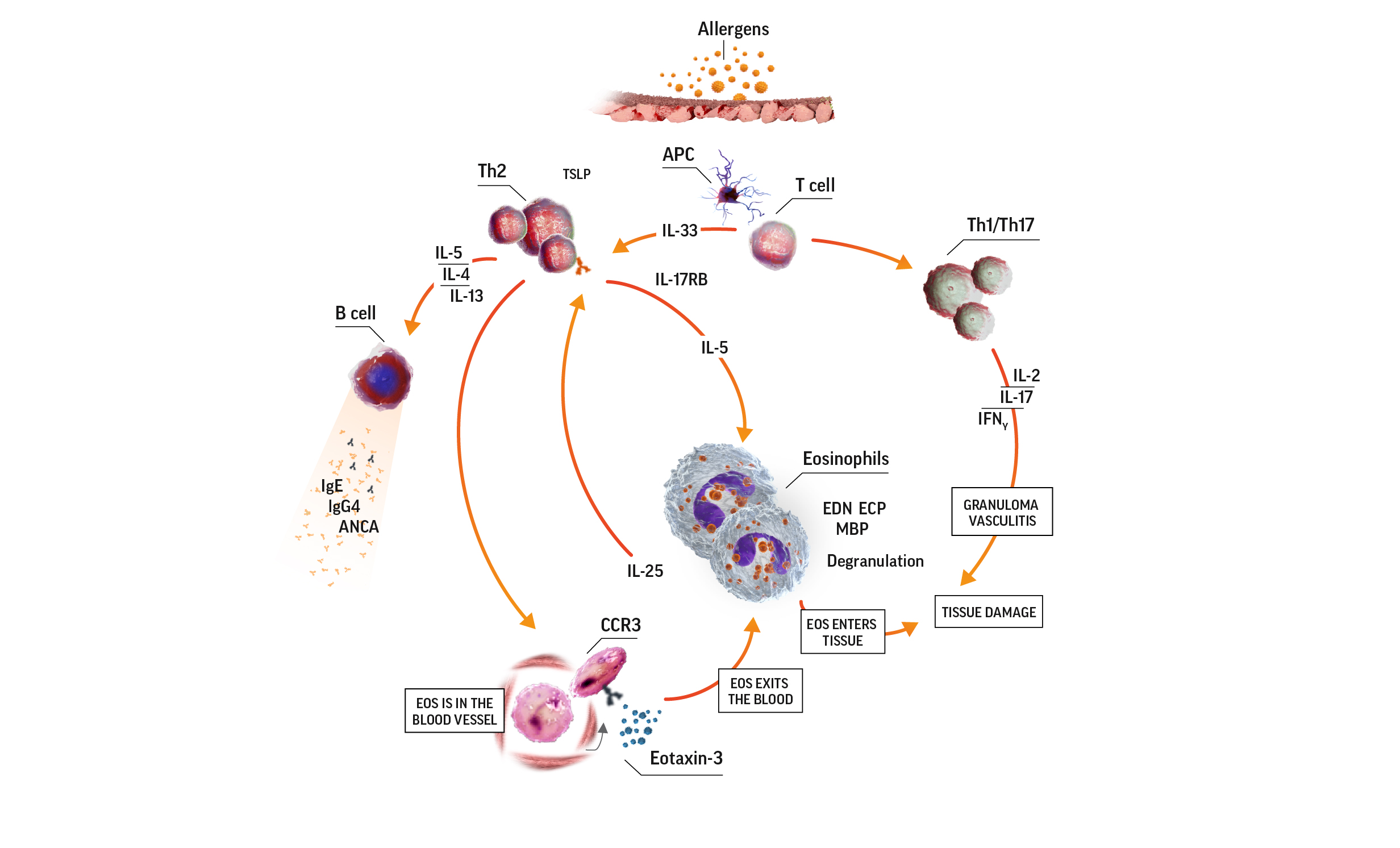
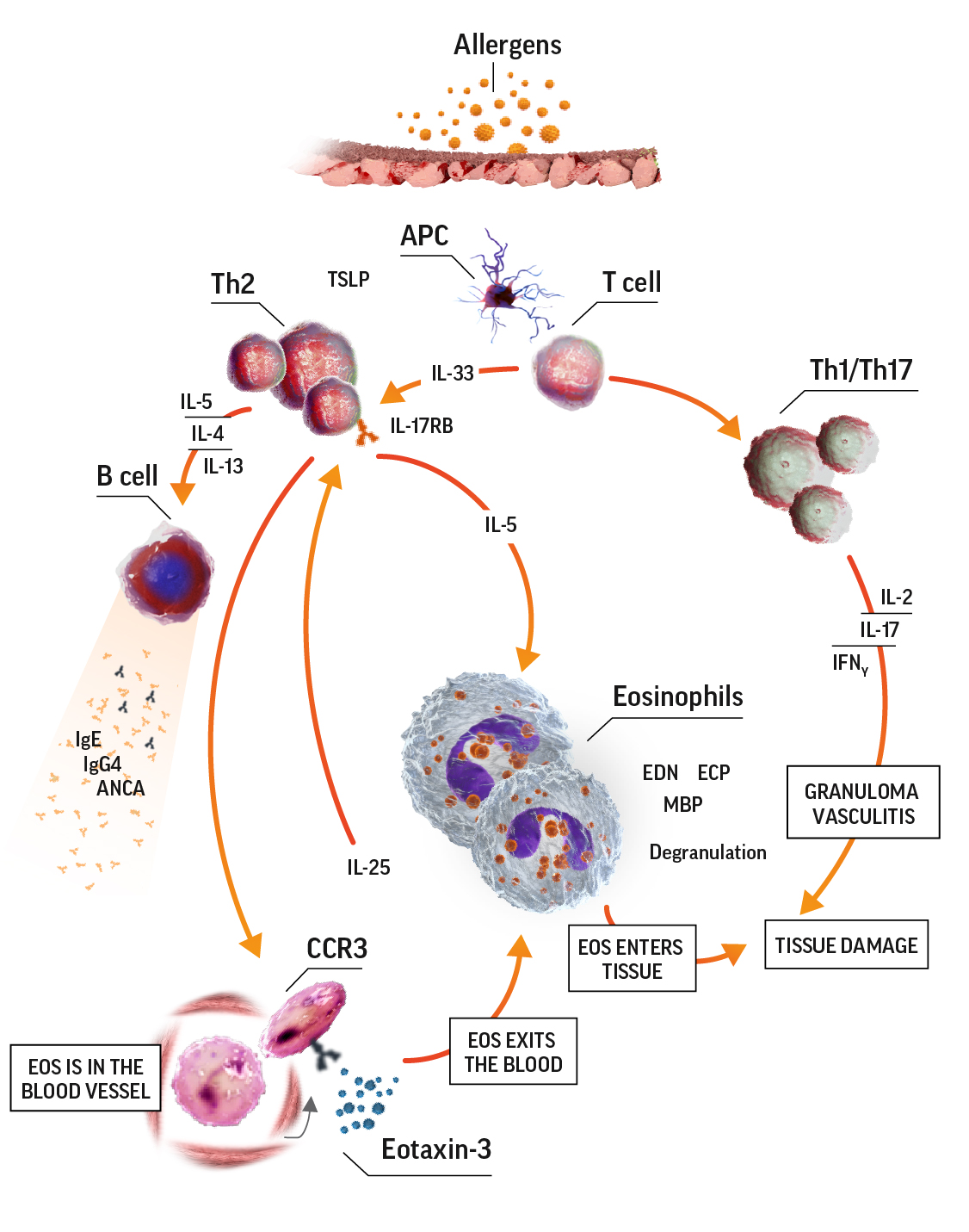
ANCA=antineutrophil cytoplasmic antibody; APC=antigen-presenting cell; CCR3=C-C motif chemokine receptor 3; ECP=eosinophil cationic protein; EDN=eosinophil-derived neurotoxin; IFNɣ=interferon gamma; IgE=immunoglobulin E; IgG=immunoglobulin G; IL=interleukin; IL-17RB=interleukin 17 receptor B; MBP=major basic protein; TCR=T-cell receptor; Th=T helper; Th2=T helper cell type 2.
Clinical manifestations and symptoms can vary across patients and depend on which organs are targeted4,10,11:
Cardiovascular
Constitutional
Dermatological
Gastrointestinal
Neurological
Otolaryngologic
Pulmonary
Renal
EOS counts in EGPA diagnosis
An eosinophil count that is >10% of a white blood cell count can help identify patients with EGPA.3
In addition to blood eosinophil counts, the American College of Rheumatology (ACR) includes additional criteria to aid in classifying EGPA. Presence of ≥4 of the below criteria yields a sensitivity of 85% and a specificity of 99.7% for EGPA3:
- Eosinophilia: >10% eosinophils in peripheral white blood cell differential count
- Extravascular infiltration of eosinophils
- Asthma
- Mononeuropathy or polyneuropathy
- Paranasal sinusitis abnormalities
- Pulmonary infiltrates (may be transient)
The ACR criteria is for classification of patients diagnosed with systemic vasculitis.
Tools for EGPA diagnosis
Laboratory and imaging tests12
- Tests are based on patient presentation
ANCA detection12,13
- ANCA is observed in approximately 40% of patients with EGPA
- ANCA negativity does not rule out EGPA diagnosis
Biopsy12,13
- Biopsy is considered the gold standard for diagnosis; however, many patients are diagnosed on a clinical basis
- Biopsy sites include skin, nerve, muscle, endomyocardium, kidney, esophagus, stomach, and/or intestines
- A biopsy that shows a small or medium vessel vasculitis and eosinophil infiltration support an EGPA diagnosis when other clinical manifestations of EGPA are present
Could it be HES?
Differentiating between EGPA and hypereosinophilic syndrome (HES) is challenging—both conditions have persistent eosinophilia and systemic manifestations.14
EGPA characteristics that differ from HES include1,7,14:
- Asthma and nasal polyps
- Vasculitic complications
- ANCA-positivity (in ~40% of patients)
ANCA-positivity, along with other factors, can aid in differential diagnosis of HES or EGPA.14
Could it be another necrotizing vasculitis?
Blood eosinophilia and asthma may help differentiate EGPA from other small- to medium-vessel necrotizing vasculitides.1,7
Explore why EOS counts in different disease states
Severe eosinophilic asthma (SEA)
References:
- Jennette JC, Falk RJ, Bacon A, et al. 2012 Revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1-11.
- Latorre M, Baldini C, Seccia V, et al. Asthma control and airway inflammation in patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract. 2016;4(3):512-519.
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990;33(8):1094-1100.
- Baldini C, Talarico R, Della Rossa A, Bombardieri S. Clinical manifestations and treatment of Churg-Strauss syndrome. Rheum Dis Clin North Am. 2010;26(3):527-543.
- Annual Estimates of the Resident Population for the United States, Regions, States, and Puerto Rico: April 1, 2010 to July 1, 2019. U.S. Census Bureau, Population Division. Accessed June 11, 2021.
- Data on file, GSK.
- Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy. 2013;68(3):261-273.
- Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol. 2014;10(8):474-483.
- Pelaia G, Vatrella A, Busceti MT, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;2015:879783.
- Noth I, Strek ME, Leff AR. Churg-Strauss syndrome. Lancet. 2003;361(9357):587-594.
- Moosig F, Bremer JP, Hellmich B, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis. 2013;72(6):1011-1017.
- Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med. 2015;26(7):545-553.
- Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65(1):270-281.
- Leurs A, Chenivesse C, Lopez B, et al. C-Reactive protein as a diagnostic tool in differential diagnosis of hypereosinophilic syndrome and antineutrophil cytoplasmic antibody-negative eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract. 2019;7(4):1347-1351.
